

CPPL Regulatory section is supported by a team of highly skilled professionals in QA QC Clinical and Production, which enhances the power and effectiveness of the Regulatory department. This is evident in the impressive results achieved. With their extensive experience in preparing over 3000 dossiers and DMFs, CPPL is recognized as the most preferred regulatory service provider in the pharmaceutical industry.
This ASEAN Common Technical Dossier (ACTD) serves as a comprehensive guideline outlining the mutually agreed upon standardized format for creating meticulously prepared Common Technical Dossier (CTD) applications, which are to be presented to regulatory bodies within the ASEAN region for the purpose of pharmaceutical registration for human consumption. This guideline elucidates the structure of the CTD and emphasizes its role in streamlining the application process, effectively minimizing the time and resources required for compiling registration applications. Furthermore, it is anticipated that this format will facilitate the future preparation of electronic submissions. The utilization of a standardized document featuring common elements will expedite regulatory reviews and enhance communication between regulatory authorities and applicants.
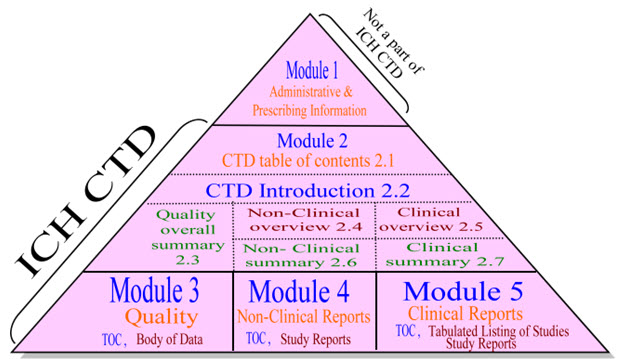
The Association of Southeastern Asian Nations, or ASEAN, has taken note of this and is now developing the ASEAN CTD, a standard that is based on the CTD. The ACTD divided the submission into 4 sections rather than the original 5 modules. This is carried out because reference applications—that is, applications to dispense drugs that have already been granted in another country—are typically the only ones that ASEAN countries receive. Consequently, there is less of a need for thorough documentation because most study reports do not need to be turned in. Part I of the ACTD still contains the regional and registration information found in Module 1 of the CTD. The summaries are incorporated into the subsequence sections and ICH M2 is removed.Quality information (ICH M3) is Part II of the ACTD, Nonclinical (ICH M4) is Part III, and Clinical (ICH M5) is Part IV.
1. PART I : ADMINISTRATIVE INFORMATION
2. Part II : Quality
3. Part III: Nonclinical Study Reports
4. Part IV : Clinical Study Reports.
Cyclone Pharmaceuticals Pvt Limited is a prominent regulatory consulting firm in India, specializing in the compilation of ACTD Dossiers through thorough document review processes. Over the past two years, our expertise has grown extensively, allowing us to successfully compile and submit ACTD Dossiers in various Asian countries. We acknowledge the practical challenges encountered in this process and are committed to providing effective solutions. Our role as dossier consultants extends beyond compilation, as we also engage in document review and correction.
Drawing from our experience in drug dossier compilation, we have observed numerous instances of inadequate and flawed manufacturer documents. Directly incorporating such documents into the dossier can result in significant queries and delays during the registration procedure. We firmly believe that compiling a dossier is not merely a task for a computer operator, but rather a responsibility of skilled technocrats who possess the ability to review documents and offer appropriate corrections to minimize queries. The regulatory team at CPPL understands this and advises clients on the technical aspects of their documents, ensuring that their ACTD Dossiers are perfected for registration.